
Arp2/3 Protein Complex (Porcine brain)
| 货号 | RP01P-A | 售价(元) | 6450 |
| 规格 | 2 x 50 µg | CAS号 |
- 产品简介
- 相关产品
Product Uses Include:
Positive control for in vitro actin polymerization stimualting compounds
Discovery of Arp2/3 activating or inhibiting compound
Characterization and discovery of Arp2/3 interacting proteins
Material:
The Arp2/3 protein complex consists of 7 protein subunits, all present in approximately equal stoichiometry (see Figure 1). The Arp2/3 complex is a key regulator of actin filament nucleation and the protein subunits are highly evolutionarily conserved. The protein complex has been purified from porcine brain and is supplied as a lyophilized powder. When reconstituted with distilled water, the complex is in the following buffer: 20 mM Tris pH 7.5, 25 mM KCl, 1 mM MgCl2, 0.5 mM EDTA, 0.1 mM ATP, 1% dextran and 5% sucrose. The molecular weight of the Arp2/3 complex is 224 kDa.
Note: RP01P from procine brain replaces RP01 from bovine brain. We have compared the activity of Arp2/3 complex from both sources and have found them to behave identically in actin polymerization assays (see Fig. 2)
Purity:
Protein purity is determined by scanning densitometry of Coomassie Blue stained protein on a 4-20% polyacrylamide gel. Arp2/3 protein complex is determined to be 90% pure (see Figure 1).
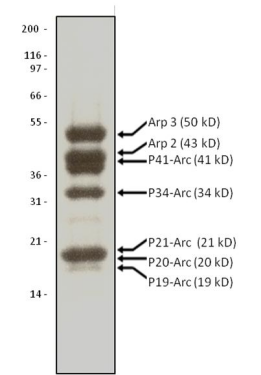
Figure 1. Arp2/3 Protein Purity Determiniation: A 10 μg sample of Arp2/3 complex was separated by electro-phoresis in a 4-20% SDS-PAGE system and stained with Coomassie Blue. Protein quantita-tion was determined with the Precision RedTM Protein Assay Reagent (Cat. # ADV02). Mark12 molecular weight markers are from Life Technologies.
Biological Activity:
RP01P was tested in an actin polymerization assay (Cat. # BK003). In conjunction with the VCA domain of WASP (Cat. # VCG03), an Arp2/3 activator, it was shown to stimulate actin polymerization by 20-fold compared to the control without RP01. This indicates Arp 2/3 specificity for actin polymerization induction (Figure 2).
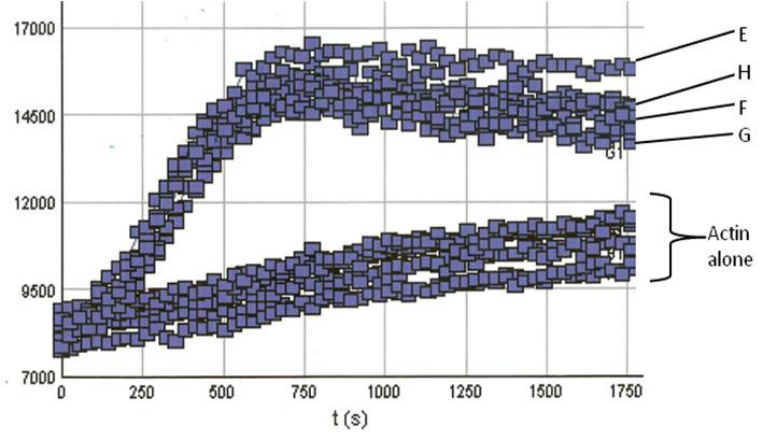
Figure 2. Actin Polymerization Assay with Arp2/3 and VCA domain proteins: Actin polymerization was carried out as described in the method; All reactions contain 0.8 μM actin. Reactions containing Arp2/3 or VCA (data not shown) show little or no enhancement of actin nucleation. Actin alone also shows low polymerization com-petence under these conditions (Actin alone reactions). In the presence of the VCA domain however, Arp2/3 results in an en-hancement of actin nucleation (Note the steep nucleation phase of polymerization). Actin polymerization is measured in arbitrary fluorescent units over time. In our tests porcine derived Arp2/3(wells E & F) behaved identically to Arp2/3 derived from bovine sources (wells G & H).


